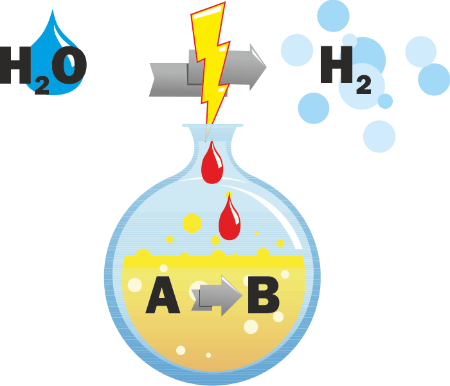
General Concept
Due to increasing concerns with respect to global warming by greenhouse gases, renewable energy resources are becoming more and more important. Electricity is one of the primary forms in which renewable energy is harvested. With a stronger penetration of renewable energy in the electricity market, it is expected that additional load to level electrical energy will be required. One possibility to store electrical energy is the generation of hydrogen via electrolysis. The splitting of water into H₂ and O₂ is the only conceivable reaction that can operate on grid scale to store the required amount of electricity and make it available later on. However, due to the easy and inexpensive access to H₂ from fossil fuels, an investment in electrolysis plants for hydrogen production is currently not competitive. Furthermore, despite the enormous advances which have been made in the past decade in the oxygen evolution reaction (OER), a significant overpotential has to be paid for a product - O₂ - which is just released into the environment. Hence, one principally simple way to make H₂ production by electrolysis economically more attractive would be the generation of value-added products at the anode. The value of the coupled, anodically generated product would subsidize the cathodically generated H₂ and vice versa.
Therefore, this consortium is devoted to the exploration of unusual anode reactions, coupled with H₂ formation in the coupled cathode compartment. We will exploit both inorganic as well as organic oxidation reactions at a hierarchically level of complexity. To address this challenging, until now largely unexplored field of research, the consortium consists of groups with a background in electrocatalysis and fundamentals of electrochemistry, organic electrochemistry, synthetic organic electrochemistry, inorganic materials chemistry, multiscale modeling and simulation, as well as heterogeneous catalysis.